NATIONAL TUBERCULOSIS PROGRAMME
National Tuberculosis programme (NTP) has been in operation since 1962
OBJECTIVES :-
Long term objectives
1. to reduce tuberculosis in the community to that level when it ceases to be a public health problem, ie
(1) one case infects less than one new person annually;
(2) the prevalence of infection in the age group below 14 years is brought down to less than 1 percent.
Operational or short term objectives
(1) to detect maximum number of TB cases among the outpatients attending any health institution with symptoms suggestive of tuberculosis and treat them effectively
(2)to vaccinate newborns and infants with BCG
(3)to undertake the above objectives in an integrated manner through all the existing health institutions in the country,
DISTRICT TUBERCULOSIS PROGRAMME (DTP)
- The program operates through the district tuberculosis program (DTP) which is backbone of NTP.over 600 TB clinics have been set up in the country ,of which 390 have been upgraded to as District TB Centres (DTC).
- DTC is the nucleus of the DTP .The function of DTC is to plan ,organize and implement the DTP in the entire district ,in association with general health services.
- The activities of DTC include case finding and treatment .The treatment is free and is offered on domiciliary basis from all the health institutions.
REVISED NATIONAL TUBERCULOSIS CONTROL PROGRAMME
The Government of India, WHO and World Bank together reviewed the NTP in the year 1992, Based on the findings a revised strategy for NTP was evolved. The salient features of this strategy are:
(1) Achievement of at least 85 per cent cure rate of infectious cases through supervised Short Course Chemotherapy involving peripheral health functionaries;
(2) Augmentation of case finding activities through quality sputum microscopy to detect at least 70 per cent estimated cases; and
(3) Involvement of NGOs; Information, Education and communication and improved operational research.
The revised strategy was introduced in the country as a pilot project since 1993 in a phased manner as pilot phase I,phase II,phase III by the end 1998 only 2 percent of the total population of India was covered by RNTCP. Large scale implementation began in late 1998.
RNTCP phase II is built upon infrastructure already established by the previous natio6 tuberculosis programme while incorporating the elements of the internationally recommended DOTS.
In 2006, STOP TB strategy was announced by WHO and adopted by RNTCP.
The components are as follows:
1.Pursuing quality DOTS - expansion and enhancement.
2.Addressing TB/HIV and MDR-TB.
3.Contributing to health system strengthening.
4.Engaging all care providers.
5.Empowering patients and communities.
6. Enabling and promoting research (diagnosis,treatment,vaccine).
In 2014, the World Health Assembly unanimously approved to end global TB epidemic by "End TB Strategy", a 20 year programme with vision of a world with zero death, disease and suffering due to TB. For details.
ORGANIZATION
State Tuberculosis Office - State Tuberculosis Officer
State Tuberculosis Training
and Demonstration Centre - Director
District Tuberculosis Centre - District Tuberculosis Officer
Tuberculosis unit - Medical Officer - TB Control
- Senior Treatment Supervisor
- Senior TB Laboratory Supervisor
Microscopy Centres, Treatment Centres
DOTS Providers
RNTCP Organogram
RNTCP structure comprises of five levels: National, state, district, sub-district and peripheral health institute levels'
NTI - National Tuberculosis Institution
NIRT -National Institution of Research in TB
DST - Drug Sensitivity Testing
SDS - State Drug Store
IRL - Intermediate Reference Laboratory
NITRD - National Institute of TB & Respiratory Disease
DRTB - Drug Resistant TB
JALMA - Japanese Leprosy Mission for Asia
NTWG- National Technical Working Group
LABORATORY NETWORK
ROLE OF EACH LEVEL OF LABORATORY
1.NATIONAL REFERENCE LABORATORTY(NRL)
- 6 CENTRES- NEW DELHI, CHENNAI,BANGALORE ,AGRA, BUNESHWAR ,BHOPAL
- Supervision of sputum microscopy EQA activitie
2.INTERMEDIATE REFERENCE LABORATORY(IRL)
- State TB training and demonstration centres or public health lab/medical college laboratory
- Conducts sputum microscopy EQA for the state
- Provides technical training to the district and subdistrict technicians and senior TB lab supervisors.
- Conducts on site evaluation visists of each DTC atleast once a year
- Manufactures slides for panel testing
3.DISTRICT TB CENTRES
- Conducted blinded rechecking nof smears
- Maintain good quality reagents and equipment at all TB units
4.TUBERCULOSIS UNITS
- At sub District levels
- Conduct on site evaluation and blinded rechecking of smears
5.DESIGNATED MICROSCOPY CENTRES
- At peripheral level
- 1 per 1 lakh population (in hilly areas 50000)
- Located at either CHC,PHC,TB dispensaries.
- Each centres has skilled technicians
- A senior TB lab supervisor is appointed every 5 microscopy centre.
RNTCP endorsed TB diagnostics :-
1. Smear microscopy for acid fast bacilli.
a. Sputum smear stained with Zeihl-Neelsen staining; or b. Fluoresence stains and examined under direct or indirect microscopy with or without LED.
2. Culture
a. Solid (Lowenstein Jansen) media; or b. Liquid media (Middle Brook) using manual semi automatic or automatic machines, e.g., Bactec, MGIT etc.
3. Rapid diagnostic molecular test
a. Conventional PCR based Line Probe Assay for MTB complex; or
b. Real-time PCR based Nucleic Acid Amplification Test NAAT for MTB complex, e.g. GeneXpert.
4. Radiography where available.
5. Tuberculin skin test.
PHASES OF RNTCP
RNTCP PHASE I (1992 -2006)
The revised strategy was introduced in the country in a phased manner as
Pilot Phase I
Pilot Phase II
Pilot Phase III
By the end of 1998, only 2 per cent of the total population of India was covered by RNTCP.
The RNTCP has expanded rapidly over the years and since March 2006, it covers the whole country.
DOTS strategy adopted by Revised National TB Control Programme initially had the following five main components:
1. Political will and administrative commitment.
2. Diagnosis by quality assured sputum smear microscopy.
3. Adequate supply of quality assured short course chemotherapy drugs.
4. Directly observed treatment.
5. Systematic monitoring and accountability.
CRITERIA OF DIAGNOSIS AND INITIATION OF TREATMENT
RNTCP PHASE II ( 2006- 2011)
To consolidate , maintain and further improve the achievement of the phase I.
Activities :-
1.Increse Access services to hard to reach areas
2.Strenthing intersectional collaboration
3. Implementation for DOTS - Plus for MDR cases
4 .Distribution of pediatrics drug box .
5.Institutional strengthening at national ,state or district level
6. Introduction of TB-HIV coordination, and communication Facilitators .
DRUG RESISTANCE SURVEILLANCE UNDER RNTCP
Aim
To determine the prevalence of anti mycobacterial drug resistance among
- New Cases
- Treated Cases
Plans
State wide DRS (Drug resistance surveillance) survey
ICMR ( Indian council of medical research)
RESULTS :- The results of these survey indicate prevalence of MDR - TB to be about 2.84 percent in new cases and 11.60 percent in retreatment cases.
DOTS -Plus
DOTS plus conceived by the WHO and several of its partners is a strategy currently under development for the management of multi drug resistant TB (MDR-TB).
Goal :- To prevent further development of MDR -TB.
Organization :- Designated RNTCP DOTS plus sites atleast one in each state with ready access to RNTCP accredited culture and drug susceptibility testing (DST) laboratory
TB HIV CO-ORDINATION to RNTCP AND NACO-"JOINT ACTION PLAN"
Objective
To reduce TB associated morbidity and mortality in TB-HIV patients
For effective prevention and control of both the disease
Areas of focus :-
The TB HIV coordination efforts focuses on
a. Sensitization of key policy makers to address the importance of TB - HIV coordination.
b. Coordination of services delivery to cross referrals
c. A joint training program for service providers involved in RNTCP and NACP.
d. use of universal precautions to prevent the spread of tuberculosis in facilities caring for HIV infected persons , and to prevent the spread of HIV through safe injection practices in RNTCP
e. joint efforts at IEC and at establishing a monitoring and evaluation system at District ,state ,and national levels to assess the coordination and treatment services for people living with HIV/AIDS
f. Active involvement of NGOs ,private practitioners and corporate sector.
ACHIEVEMENTS OF RNTCP
1.Treatment success Rate increases
2. Death Rate decreased
3.Involvement of NGOs,
- Private practitioners
- Medical college
- Peripheral Laboratories
- Designated microscopy centres
- Public health care providers
4. 4 URBAN DOTS projects ( Mumbai , Hyderabad, varanasi, chennai)
5.National frame work for joint TB-HIV collaborative activities by central TB division and NACO
NEW INITIATIVES :-
1. NIKSHAY: TB surveillance using case based web based IT system
Central TB Division in collaboration with National Informatics Centre has undertaken the initiative to develop a case based web based application named Nikshay. The word is combination of two Hindi words NI and KSHAY, meaning eradication of TB.
This software was launched in May 2012 and has following functional components.
1.Master management
2.User details
3.TB Patient registration and details of diagnosis, DOT provider, HIV status, follow-up, contact tracing, outcomes.
4. Details of solid and liquid culture and DST, LPA ,CBNAAT details.
5.DR-TB patient registration with details.
6.Referral and transfer of patients.
7.Private health facility registration and TB notification.
8. application for TB notification.
9.SMS alerts to patients on registration.
10.SMS alerts to programme officers.
11Automated periodic reports:
a. Case finding b. Sputum conversion c. Treatment outcome.
The programme has started using IT enabled adherence tools like 99 DOTS for HIV-TB patients. This will be expanded to all TB patients with implementation of daily regimen .
2. TB Notification
In order to ensure proper diagnosis and management of TB cases, and to reduce TB transmission and the emergence and spread of MDR-TB, it is essential to have complete information of all TB cases. According to the Government of India notification dated 7th May 2012, it is now mandatory for all healthcare providers to notify every TB case to local authorities i.e. District Health Officer/Chief Medical Officer of a district and Municipal health officer, every month in a given format .
3. Ban on TB Serology
The serological tests are based on antibody response, which is highly variable in TB and may reflect remote infection rather than active disease. Currently available serological tests are having poor specificity and should not be used for the diagnosis of pulmonary or extra-pulmonary TB. Their import, manufacturing, sale, distribution and use is banned by the Government of India .
4. Direct benefit transfer schemes
Direct beneficiary transfer systems are being established by linking TB patients reported in NIKSHAY with AADHAR and PEMS to effectively deliver benefits to TB patients and their providers .
DBT at notification and then during treatment
(DBT=Direct Benefit Transfer i.e. money credited directly to the beneficiary’s account)
– Rs. 500 each month of treatment and
– Up to Rs. 1000 as an advance.
NEWER INITIATIVES FOR TREATMENT :-
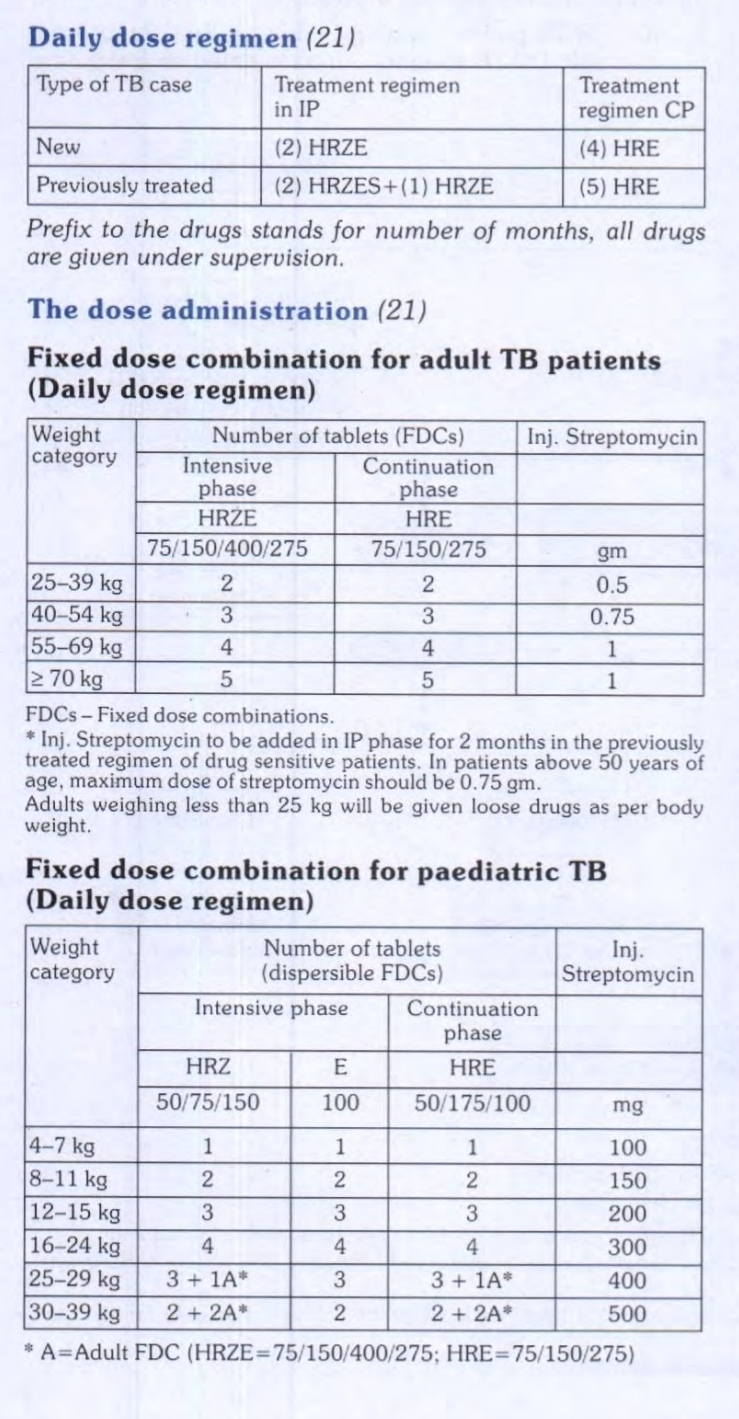
1. Daily regimen for pediatrics TB :- The current WHO dosing guidelines, the government has decided to introduce a daily dosing regimen using child-friendly fixed dosage combinations (FDCs). The procurement of anti-TB drugs in daily fixed dose combination (FDC) has been initiated. Treatment with FDCs of anti-Tb drugs will be in six weight bands for paediatric patients. An option for family members to provide Directly Observed
2. Daily regimen for all forms of TB in the country
3.Pilots for universal access to TB cases
4. Bedaquiline conditional access Programmes
5.Campaign mode :- Active case finding: To reach the unreached, the programme has carried out systematic active TB screening among high risk populations through house visits or targeted setting visit (tribal population, slums, old age homes, prisons, orphanages, transit camps etc.) The campaign was conducted in priority districts selected based on burden of TB, case finding efforts, HIV-TB and drug resistant TB in the respective districts
National Strategic Plan (2017-2025) for TB Elimination
The National Strategic Plan (NSP) 2017-2025 for TB elimination builds on the success of last NSP It is a three year costed plan and an eight year strategic document. It provides goals and strategies for the country's response to the disease during the period 2017-2025 to bring about significant changes in the incidence, prevalence and mortality of TB, and attain the global End TB targets five years ahead of Sustainable Development Goal of TB free India.
The VISION is - TB free India with zero deaths, disease and poverty due to TB
Objectives:
The main objectives of NPS are:
1. Find all drug sensitive TB and drug resistant TB cases with an emphasis on reaching TB patients seeking care from private providers, and undiagnosed TB in high-risk populations.
2. Initiate and sustain all patients on appropriate anti-TB treatment wherever they seek care, with patient friendly systems and social support.
3. Prevent the emergence of TB in susceptible populations.
4. Build and strengthen enabling policies, empowered institutions, additional human resources with enhanced capacities, and provide adequate financial resources.
The key strategies are as follows:
1. Private sector engagement
2. Active case finding
3 . Drug resistant TB case management
4. Addressing social determinants including nutrition
5. Robust surveillance system
6. Community engagement and multi-sectoral approach
Expected outcome:
The aim of the National Strategic Plan is to achieve elimination of TB by 2025. During plan period, targets for TB are:
1. 80% reduction in TB incidence (i.e . reduction from 211 per lakh to 43 per lakh)
2. 90% reduction in TB mortality (i.e. reduction from 32 per lakh to 3 per lakh
3 . 0% patient having catastrophic expenditure due to TB.
























0 Comments